
What impact have past famines had on the health of modern populations?
November 17, 2022

- Related Topics:
- Gene expression,
- Environmental influence,
- Reproduction,
- Epigenetics,
- History
Mary from Australia asks:
“What is the impact that past famines have on the health of modern populations? I am particularly interested in the way the so called Irish Potato Famine has on people of Irish descent and if they have specific health issues that could come with their genetic inheritance.”
The Great Famine (1845-1849) was a period of starvation and diseases, which decreased the Irish population by 2-3 million people due to a combination of both death and emmigration1.
Although there was a large demographic change, there was minimal impact on the genetic structure of the country,2 and no major impacts have been identified. But whether there are subtle genetic effects that persist is difficult to say. This famine happened so long ago that it is difficult to study directly.
However, there is a more recent famine that has been studied in more detail (the Dutch Hunger Winter, 1944-1945). Studies related to that event suggest that while famines may not impact the DNA of a population directly, babies born during times of famine may use their DNA differently.
To understand this better, let’s discuss how we can change how we use our DNA without changing our DNA sequences.

Using what is necessary
All of our cells in our body have the same DNA. But how can all the different cells in our body (like heart, brain, or skin cells) look so different? This is because cells can control which genes they choose to “turn on or off”.
Our DNA is like the instruction manual for our body. DNA instructs our body on how to make things (like proteins) which allows us to function in life. Just like how we may not read all of an instruction manual at the same time, we don’t use all of our DNA at once. Our cells only read the parts of the manual they need. This process is called gene regulation.
Adding stop signs to our genes
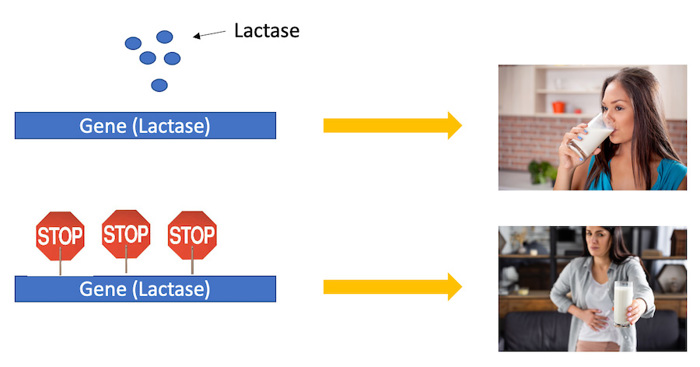
So how exactly do cells know which genes to use? One of the ways they can turn genes on or off is by putting a small chemical mark onto DNA. This mark acts like a stop sign to slow down the activity of the gene. The more stop signs there are, the more likely the gene is to turn off.
One example of this is lactose intolerance. Most people can drink milk when they are young (lactose tolerant), since milk is important for growth during childhood. However, as many people get older they are no longer able to digest milk. This happens because the gene (lactase) that helps digest milk gets turned off with age. The cells put chemical stop signs around this gene and become unable to use it.
This process of putting chemical marks on DNA is called epigenetics. While some epigenetic changes happen during puberty or adulthood (like lactose intolerance), most epigenetic changes actually happen in early development (when we are in our mother’s womb).
It is important to note that these chemical marks do not change the DNA sequence itself. And while we have our DNA forever, the epigenetic marks aren’t necessarily permanent. Many of them are reversible, or change throughout our lives. And because these marks can change, they aren’t necessarily passed down from parent to child (although this is still an area of active research).
Famines and our epigenetics
Okay, now that we have covered some of the basics, let us go back to the main question. Do famines affect our genes or how they are used?
Unfortunately, it’s a little hard to study exactly what happens during a major famine, and I was unable to find many studies on The Great Famine.
But there have been a few studies from the Dutch famine of 1944-1945. Specifically, scientists have looked at people born during the famine, to see if malnourishment during pregnancy had an impact3,4. Unborn babies are uniquely sensitive to environmental stresses since it is a time period where they are growing rapidly, and scientists wondered if they might have long term health effects.

Now, let's talk about what they found! The studies looked at people who were born during the Dutch famine, and compared them to their siblings who were born just before or after this time. And they found that people born during the famine had higher rates of type 2 diabetes3,4, cardiovascular disease3, and schizophrenia5.
Why is there such an increase in risk for those individuals? In this case, scientists found that genes involved in metabolism had different epigenetic marks4, which could explain this increased risk to diseases related to metabolism. The causes for increased risk of the other diseases are still being actively studied.
As proper nutrition is extremely important for a developing baby, it is not surprising that starvation could have such a strong effect on metabolism-related genes. These changes in metabolism may have been extremely important for a baby during this time, to help them use the limited nutrients efficiently enough to survive until birth.
Could famines affect future generations?
While research has shown that famine can have an effect on individuals born during that time, it is still uncertain whether or not those epigenetic effects are ever passed onto later generations.
A famine directly affects two generations: the parent and their unborn child. It might also have an effect on a third generation: even as an unborn baby we already have some cells that will eventually turn into either sperm or eggs, so it’s possible that a famine could directly affect that generation as well.
However, scientists think it’s unlikely that these epigenetic changes would persist to a fourth generation (the great-grandchild of the person who was alive during the famine). Since most epigenetic marks are non-permanent, it’s unlikely that they would last for so long. The genome is fixed, but our epigenome is flexible and adaptive!
Read More:
- CDC: What is epigenetics?
- Jackson Labs: What is epigenetics (in a minute)?
- History.com: The Great Irish Famine
- NYT: The effects of the Dutch Hunger Famine

Author: Christy Luong
When this answer was published in 2022, Christy was a Ph.D. candidate in the Department of Chemicals and System Biology, studying the mechanisms of random monoallelic expression in Howard Chang's and Joanna Wysocka's laboratories. Christy wrote this answer while participating in the Stanford at The Tech program.
 Skip Navigation
Skip Navigation
