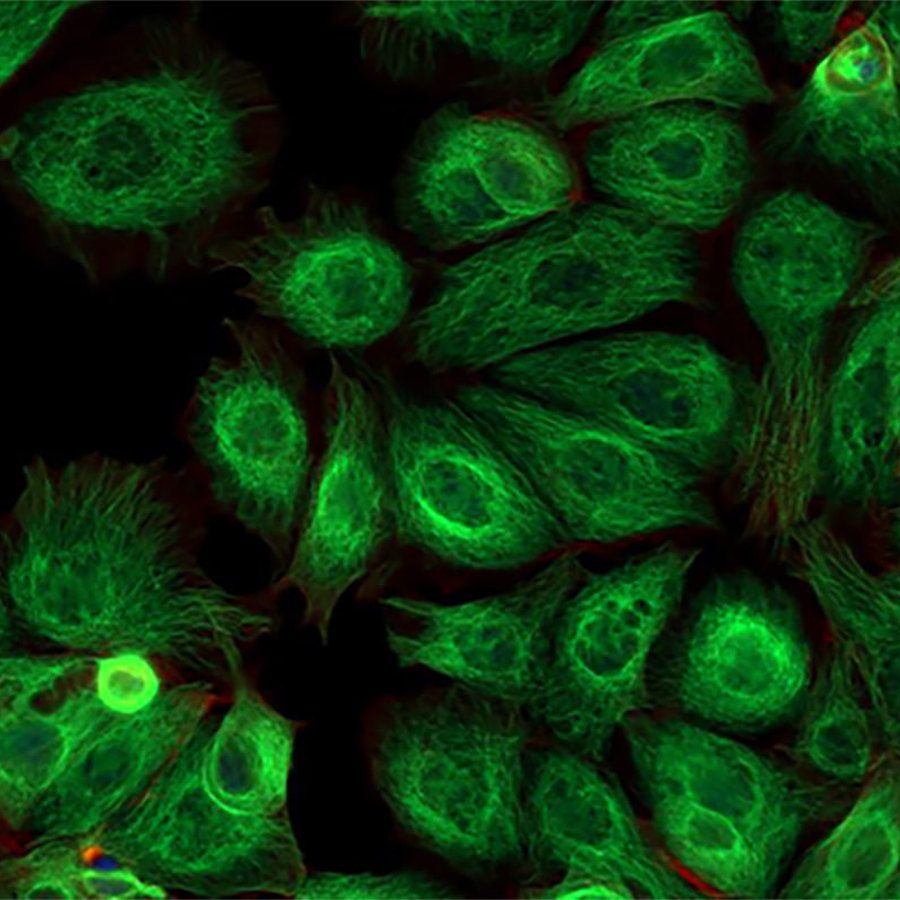
How do you design glowing cancer cells?
April 22, 2014

- Related Topics:
- Genetic engineering,
- Cancer,
- Biotechnology
A high school student from Utah asks:
“I went to a genetics workshop and they talked about altering the genes, in a cat with cancer for example, so that the cancer would glow and they could observe where it traveled. How does that work? How would you get the luminescent DNA into all of the cancer cells?”
I have not heard much about people putting a gene for a glowing protein like GFP into the cells of a cancer that was already in an animal. You are right in thinking that most likely very few of the cancer cells would take up the GFP gene.
One way they might be able to track a cancer already growing in an animal is to use antibodies that only recognize cancer cells. If they attach those antibodies to GFP, then they’d be able to see those cells in the animal. This is very different from adding the GFP gene to a cancer cell though.
When a scientist wants to use a GFP gene in a cancer cell, what they usually do is add the GFP gene to a cancer cell growing in a dish. After that they add the cancer to the animal. Now every cancer cell will be glowing. This procedure is pretty similar to what we do at The Tech Museum in the wetlab.*
In mice they can follow the glowing cancer cells while the mouse is still alive. This lets the scientists learn which cancers tend to spread where. And maybe allow for them to come up with ways to stop it from spreading at early stages. Since cancer spreading (or metastasis) is the main reason people die from this disease, this is obviously important research!
Now this was a very simple explanation of how this works. As usual, the details are a bit more complicated.

GFP in Cells
The first step is to get the cancer cells to glow green. You need different DNA for this than what we use to make bacteria glow in the wetlab.
Now you don’t need a different gene. The instructions for making GFP are pretty much the same no matter what beast is reading it. Most every living thing uses the same code to turn a gene into a protein.
What different organisms don’t do the same is recognize where a gene starts. Heck, even different types of cells from the same plant or animal can’t recognize some of their genes as genes.
So the first step is really to put the GFP gene in front of a piece of DNA that marks it as a gene in a cancer cell. This piece of DNA is called a promoter. Scientists often use viral promoters because they are strong (they will cause lots of GFP to be made) and they tend to work in many different kinds of cells.
Now we have a piece of DNA that will cause our cancer cell to glow. The next step is to make sure that every cancer cell we put into the animal glows and that they keep glowing as they grow and divide. To do this, we need to get the DNA to insert itself into the DNA of the cancer cells.
The DNA we add in the experiment we do in the wetlab doesn’t insert itself into the bacterium’s DNA. Instead it floats in the cell as its own sort of independent chromosome.
We can get away with this because in addition to the GFP gene, we also add a gene that lets the bacteria survive antibiotics. Only those bacteria that get our gene will live. If we remove the antibiotic, the chromosome is lost and the cells stop glowing.
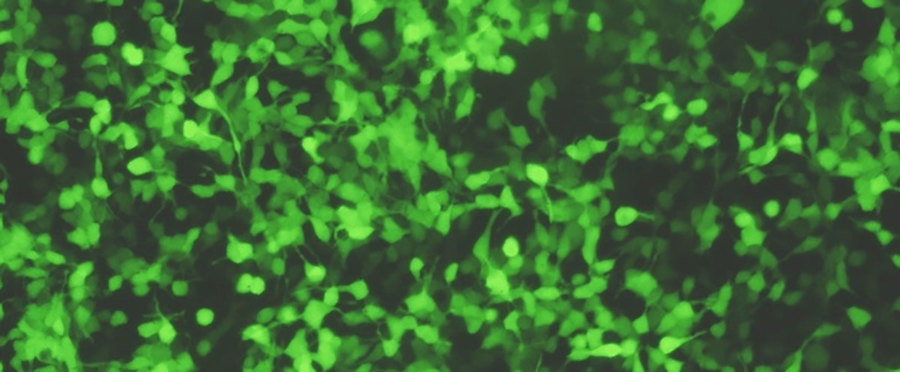
Getting DNA into a cell’s genome is not easy. To pull this off, scientists often use something called a retrovirus. These are viruses like HIV (the virus that causes AIDS) that insert their DNA into the host cell’s DNA.
Scientists remove all the deadly genes from the virus and replace them with the genes they want to use. In this case, they’d remove the deadly gene(s) and replace them with GFP.
There are lots of other ways to get the DNA in there as well. One of the most exciting new ways is called CRISPR. If it works out like everyone is predicting, it should make it easy to put the gene in wherever we want.
Once they get the DNA into the cells, they then pick the ones that actually glow and get rid of the ones that don’t. They can do this manually or with a technique called Fluorescent Activated Cell Sorting (FACS), which uses a machine to automatically separate glowing from non-glowing cells. We now have our starting material.
GFP in the Animal
The next step is to get the glowing cancer into the animal. A common way to do this is by directly injecting the cells into the animal.
An important point here is that you need to use an animal that has a terrible immune system. Otherwise your cancer will simply be targeted and destroyed before you can study it.
The usual way to do this is with something called nude mice. Yes they are hairless but more importantly they don’t have a thymus (or not a very good one anyway). This means they don’t have much of an immune system either which means we can study human cancers in these mice.
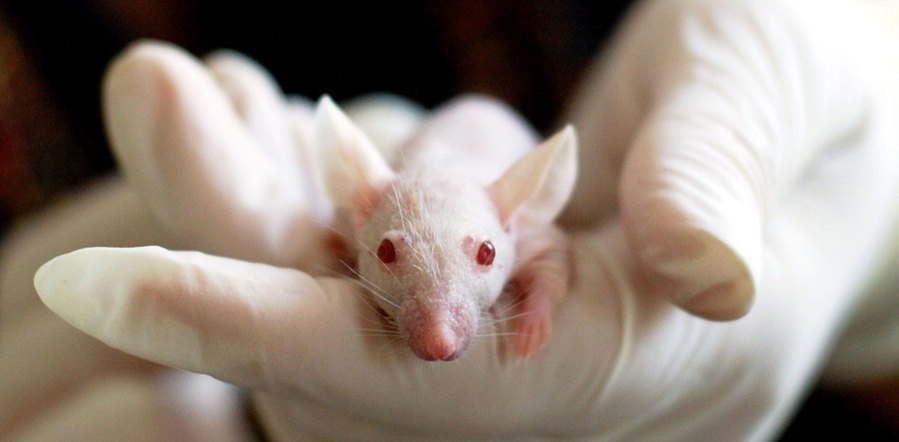
Now we are finally ready to do our experiment. We put our glowing cancer cells into a nude mouse and see how the cancer spreads.
These kinds of studies are used all the time to figure out how certain types of cancers spread. Once scientists have figured that out, they can develop medicines that block that process. Then they can stop that type of cancer from spreading and save innumerable lives.
* Editor’s note: The Wetlab was replaced in 2016 with “Living Colors Lab”, a part of the BioDesign Studio exhibit. This lab experience is very similar, except that visitors can make multicolored bacteria instead of just green.

Author: Dr. Barry Starr
Barry served as The Tech Geneticist from 2002-2018. He founded Ask-a-Geneticist, answered thousands of questions submitted by people from all around the world, and oversaw and edited all articles published during his tenure. AAG is part of the Stanford at The Tech program, which brings Stanford scientists to The Tech to answer questions for this site, as well as to run science activities with visitors at The Tech Interactive in downtown San Jose.
 Skip Navigation
Skip Navigation
