
If we knew everything about genetics, what could we do with current genetics technology?
October 16, 2014

- Related Topics:
- Quirky questions,
- Futuristic science,
- Genetic engineering,
- CRISPR
A graduate student from California asks:
"Suppose we have a geneticist who knows everything about genetics (how she got that knowledge isn’t important to this question). She doesn’t just know everything we know now but everything we could possibly know. How much genetic engineering would she be able to do with our current level of technology?"
Geneticist Gill wakes up one morning and suddenly knows everything there is to know about genetics. Awesome! But what does it mean to know everything about genetics?
First off, she will know every letter in your DNA. Not just all the letters and what order they’re in, but also what all the genes do, and when they’re on or off.
She would know your genome in incredible detail. But there is surprisingly little she could do with that information right now.
Gill would know which genes are related to which diseases. From this, she could tell you which diseases you are likely to get.
She could also look at a fertilized egg and have a good idea about what it would look like as a person. Additionally, she could see how everyone is related to everyone else, and fill in the world’s family tree.
However, even if she knew that you or the fertilized egg had a broken gene, she probably wouldn’t be able to do much to help. In the fertilized egg, she might be able to make one or two genetic tweaks to limit whatever the worst diseases might be.
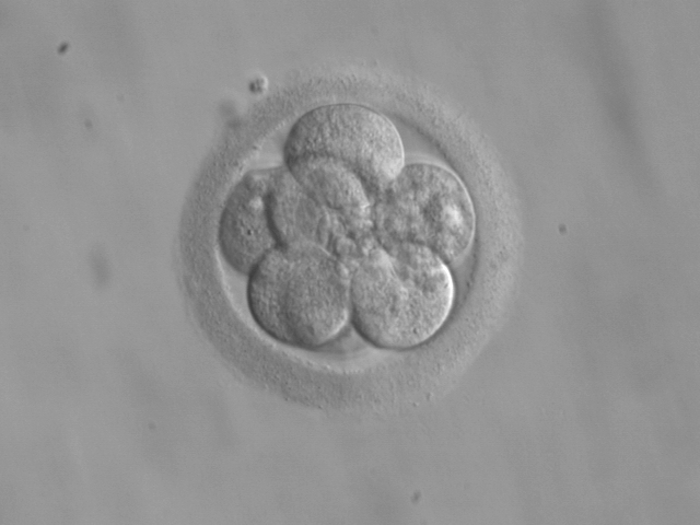
For you, her options would be much more limited. Chances are, you would have to live with whatever your genes contain, be it a heightened chance for heart disease or Alzheimer’s. She would be all knowing, but almost powerless.
But that’s just human health — there’s a whole world of biology out there that Gill could tinker with! She could engineer bacteria or yeast to make new medicines. She could engineer algae to make biofuels to help deal with global warming. She could engineer plants to make bigger, better food to feed our growing population. Or a thousand other things that I haven’t even thought of!

Genetic Engineering
Genetic engineering is when you change an organism’s genome by taking out or changing the genes they were born with. It can also mean putting in new genes.
Until recently, we didn’t have a lot of ways to do this. This has changed in the last few years.
The most extreme examples of using today’s technology to modify genomes come from the field of synthetic biology. Synthetic biologists aim to create new types of life in the lab.
For example, in 2010, scientists took the genome out of a bacteria and replaced it with a genome that they made. This year, another group created a whole new chromosome in yeast. This opens the door to reprogramming life to do what we want.
Another example from the cutting-edge of today’s genetic technology is called “CRISPR-Cas9.” This system allows scientists to change DNA sequences very precisely. It is faster and easier to use than previous technologies.
This all sounds great, but it isn’t yet as powerful as it seems. There are technical limits to what we can do today.
First of all, we don’t know how to add or delete genes in every type of organism. This makes it tough, for example, to coax algae into making fuels for us.
And as exciting as CRISPR is, it also still has major limitations. We can’t currently use it to edit the DNA in all of your cells at once, so it would be hard to fix diseases in adults. It is also inefficient in fertilized eggs, and no one has yet used it to add (rather than delete) a gene to a person (although they have added genes to flies).
And while CRISPR could technically be used to alter a human, that comes with a whole host of ethical issues! This summer, the first ever clinical trial using CRISPR was approved. And it’s for a cancer treatment in adults. Scientists pretty universally agree it’s not something that should be used on embryos yet.
Our current technology couldn’t do much with Gill’s vast knowledge. But that wouldn’t be the case forever. In fact, Gill’s knowledge would almost certainly spur new technologies.
Positive Feedback Cycle
In science, new knowledge leads to new tools. And those new tools uncover new knowledge.
Suppose all of the possible knowledge about genetics fit in a box. Let’s say that it is this big:

Now let’s look at the box holding all the current knowledge about genetics:

It’s much smaller. We’ll need a bigger box for all the knowledge we will gain in the future!
How do we make a bigger box? We need new tools. Once we have new tools (bigger pieces of wood, longer nails, stronger hammers, etc.), we can make a bigger box, and fill it with lots of new knowledge.
This is often how things work in science. Our new tools are new technologies, like faster ways to read genomes or more precise ways to change a gene.
The larger “box” is the ability to ask new questions and answer them with the new technologies. This is an iterative process. That means that new knowledge leads to new technologies, which lead to new knowledge, and so on.
For example, the CRISPR technology that I mentioned earlier came from learning new things. It was inspired by how immune systems work in bacteria. Now we can use this technology to answer questions that we couldn’t answer before.
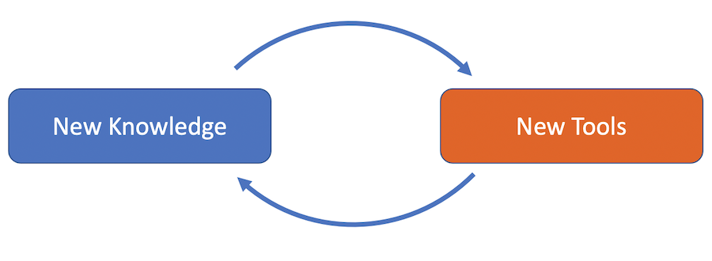
Gill’s case is different. She suddenly knows more than our current technology could tell her! Now, her new knowledge can push the limits of technology by leaps and bounds.
This could happen when you get your knowledge from somewhere else, like a different field of science, or in Gill’s case, mysterious magic. But it throws off all our predictions about what Gill would be able to do.
By merely knowing more about genetics, Gill will be able to do more. Many of the current limitations to our tools exist because we don’t know enough about how they work. But Gill will know more about how they work, so those same tools will be more powerful in her hands!
Nobel Prize winning physicist Richard Feynman said, “What I cannot create, I do not understand.” Gill could find herself dealing with the inverse of this: what I understand, I can create!
Beyond technology
Up until now we have dealt with how technology could get in Gill’s way. But there are also other things that could limit Gill.
One area that could give Gill problems is money. Many of her goals may be technically possible, but just too expensive.
For example, the synthetic bacterial genome I mentioned earlier cost about $40 million to make! Most labs don’t have pockets quite so deep.
She would also have to consider ethics. Just because something is possible, doesn’t mean it should be done.
For example, there is a new technique that lets women with faulty mitochondria have children who don’t inherit those genetic defects. But since this method combines DNA from three different individuals, it is controversial. This practice has been banned in the U.S. because of the ethical concerns about children with three parents. (Click here to learn more about the controversy; click here to meet a girl who was conceived this way.)
Finally, Gill will have to know more than just genetics to fully understand what she’s doing. Genetics is like a blueprint for a house. But there’s a lot that needs to happen between having a blueprint for a house and actually living in it. You can know everything there is to know about the blueprint, but you still need to build the house.
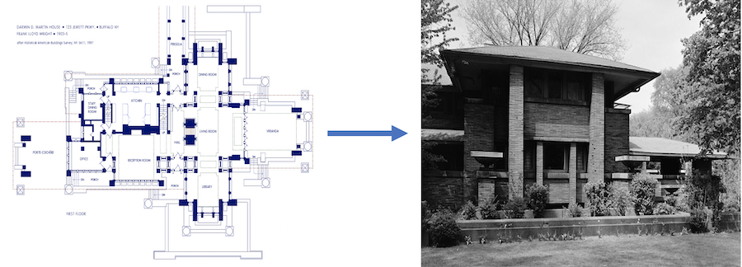
To actually build the house, you need to know about carpentry, electrical engineering, plumbing, and more. Just like in biology, to really use “everything there is to know about genetics,” we need to know a lot about what happens between the genes (“blueprint”) and the living organism (“house”). That’s biochemistry, molecular and cellular biology, physiology, and more.
That’s the beauty of biology: it’s all interconnected.

Author: Liz Freeman
When this answer was published in 2014, Lucy was a Ph.D. candidate in the Department of Biomedical Informatics, studying gene expression changes in mammalian aging in Stuart Kim’s laboratory. She wrote this answer while participating in the Stanford at The Tech program.
 Skip Navigation
Skip Navigation
